What's The Difference of Adiabatic & Isothermal Process?
Adiabatic Process and Isothermal Process are common terms of thermodynamic while discussing the energy variation in form of heat. To understand the difference of adiabatic process and isothermal process, one can start from the definition of Carnot Heat Engine. In this article, ACTTR Technology brought to you the relate topics and gave you some ideas of the principles of adiabatic process and isothermal process, follows by the below sections:
What Is Carnot Heat Engine?
What Is Adiabatic Process?
What Is Isothermal Process?
Carnot Cycle
Thermal Analyzer and Calorimeter
What Is Carnot Heat Engine?
"Carnot Heat Engine" is used to describe a virtual model of a thermodynamic system in which the endothermic and exothermic process is performed. A thermodynamic system sources the heat from a hot reservoir, and then sinks the heat to a cold reservoir. In the meantime, it has for processes:
- Isothermal Expansion
- Adiabatic Expansion
- Isothermal Compression
- Adiabatic Compression
The following figure is a typical Carnot Heat Engine. The middle yellow mechanism is the thermodynamic system, which is equipped with fluid working substance (liquid, gas, steam) for participating the process. The red hot reservoir at the left side sources the heat to the thermodynamic system, and the blue cold reservoir at the right side sinks the heat from the thermodynamic system. We assume both of the reservoir have infinite capacity of supplying and absorbing the heat to/from the thermodynamic system.
(Note: We will not consider the reversible conditions in this article to avoid too complicated factors. For more detailed introduction, please refer to other professional articles or papers.)
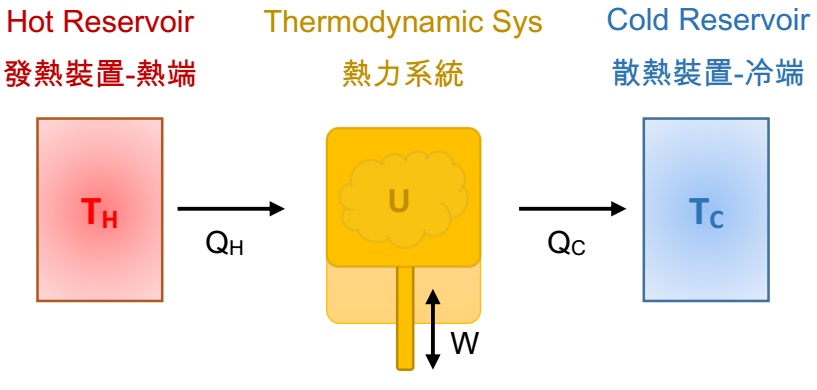
- TH: absolute temperature of the hot reservoir.
- TC: absolute temperature of the cold reservoir.
- QH: the heat sources from high temperature hot reservoir during isothermal expansion process.
- QC : the heat sinks to a low temperature cold reservoir during isothermal compression process.
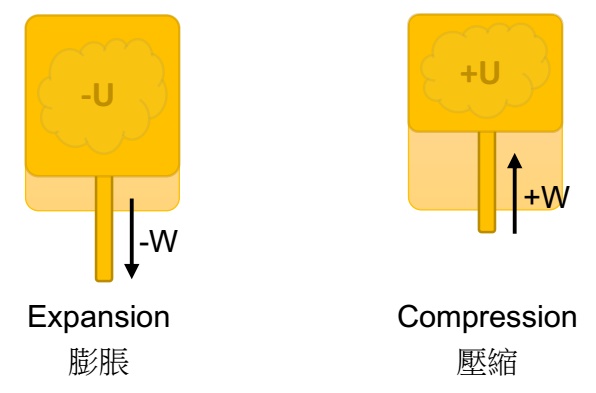
- W: the work done by the thermodynamic system (adiabatic expansion) or by the external environment (adiabatic compression).
- U: internal energy of the thermodynamic system
What Is Adiabatic Process?
During the operation of a thermodynamic system, there is no energy in the form of heat get in and out of the system from the external environment. There is only internal energy (temperature) variation, and the energy is converted into mechanical energy, which can be expansion (work done by the thermodynamic system) or compression (work done by the external environment). The internal energy and the mechanical energy can be represented by ΔU = W. Although the transmission of heat is isolated in adiabatic process, the energy (temperature) within the system temp is not constant.
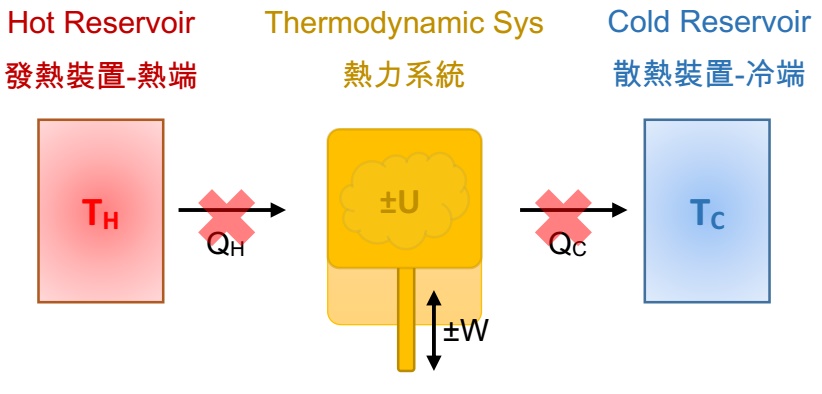
What Is Isothermal Process?
During the operation of a thermodynamic system, the system maintains a constant internal temperature, say, ΔU = ΔT = 0.
But the external heat transmission with the system exists, heat is absorbed or released by converting it into mechanical energy directly, such as isothermal expansion (work done by the thermodynamic system) or isothermal compression (work done by the external environment). The energy transmission can be represented by Q = W.
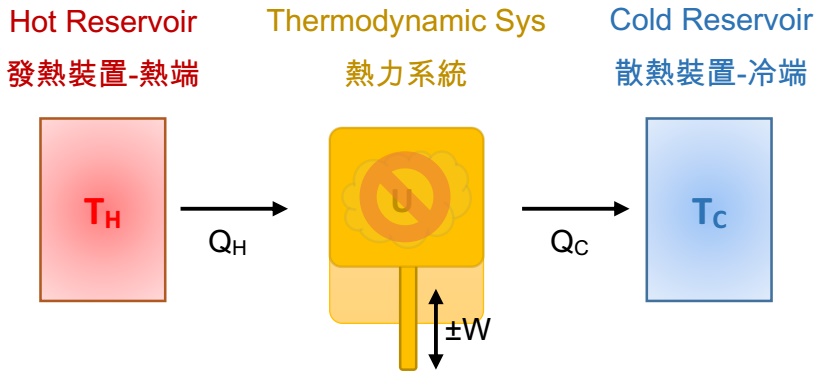
Carnot Cycle
Conclude the above concepts, the four steps of a Carnot Heat Engine can be considered in a PV diagram, with the factors of pressure volume and temperature. It will be easier this way to understand the Carnot Cycle and the four processes:
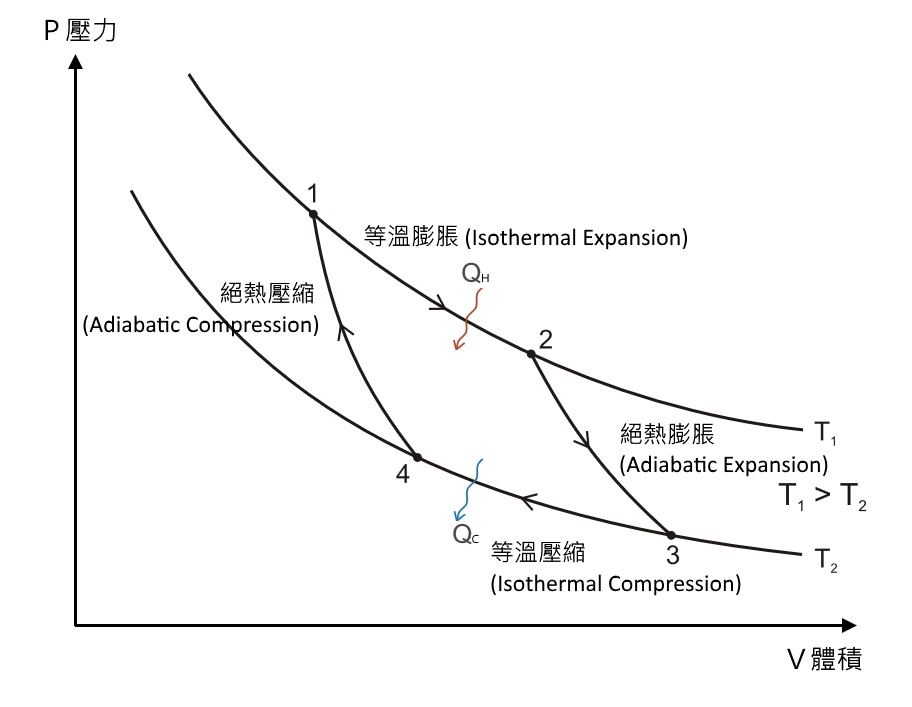
The two curves T1, T2, represent the relative variations of pressure and volume of fluid substance in the thermodynamic system under a constant temperature. (enlarge the pressure, volume decreased). In the below figure, the temperature of T1 curve is larger than T2 curve.
In the PV diagram Carnot Cycle, one can understand how the heat of QH and QC are interchanged during the isothermal expansion process and isothermal compression process. Therefore, even the pressure is changed, the system can still maintain a constant temperature (moving along the known P-V curve of a constant temperature). The volume of substance expanded, the pressure is reduced; the volume is compressed, the pressure is increased.
In the process of adiabatic compression and adiabatic expansion, there is no external heat, Q, get in or get out of the system. The relative change in volume and pressure are the same, however, in the expansion process, the internal energy decrease and lower the temperature. In the compression process, it’s opposite. The temperature is raised.
Thermal Analyzer and Calorimeter
In the real world, the above ideal curves are not existed. In order to observe the real conditions and the relative reactions among pressure, volume, and temperature, you need a good calorimeter or thermal analyzer. And this equipment can help you to build up the conditions of a isothermal or adiabatic environments.
ACTTR Technology brought to you the most advanced 3D Calvet DSC Calorimeter from SETARAM. It has the world biggest sample volume, highest temperature, and a huge types of sample cells for your various experiments and researches. Please find ACTTR for more details!





