How A XRF Analyze The Elements?
Atomic electron can be ejected out of the original orbital by absorbing the X-ray energy, and this energy must be greater than the energy that the electron is bound to the nucleus of the original orbital. When this inner-shell electron is ejected out of the orbital, one another electron in the outer shell, with higher energy, will fill out this empty position, and the release the energy in the form of photons (fluorescence), and the energy is exactly equal to the energy difference between the two neighborhood shells.
Such kind of fluorescence that is generated by the electronic transitions, is so called characteristic X-ray of the element. Since the energy dissipation between two adjacent orbitals is always a constant, by measuring the amount of fluorescence can identify a particular element. It is the principle of the X-ray Fluorescence Spectroscopy (XRF). And the spectrometer is designed.
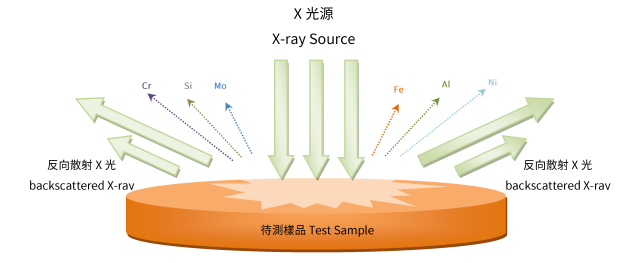
Principle of XRF
Furthermore, a particular fluorescence of an element, of which the number of photons per time unit is related to the materials in the sample. Typically, X-ray Fluorescence Spectroscopy (XRF) represent the value by a peak intensity or count rate. This result will be presented in the characteristic X-ray lines from the spectrometer. Each element has its peak value belongs; and these fluorescent spectrum lines will appears in the shape of the semi-Gaussian distribution, because the count of the resolution of the modern detectors are still not perfect .
Therefore, the observation of these peaks, and the number of the peaks of elements, we can have the composition of elements in the material. Besides, by quantitatively measurement, one can analyze the concentration of samples and have better understanding on the composition of the entire sample by a spectrometer.
ACTTR is the most aggressive & the most professional instrument of spectrometer distributor, agent, reseller, in Taiwan.


