Principle of Pressure / Vacuum Measurement & Units
Pressure and vacuum are used in many scientific instruments, they are typically the measurement results or the control factors. e.g. Fluid Sorption Tester, Thermogravimetric Analyzer, Gas Analyzer, High-pressure Diamond Anvils, etc. In addition to common pressure gauges and vacuum gauges in these test instruments, pressure pumps or vacuum pumps are also used to create the necessary pressure or vacuum conditions. There are various pressure unit in a pressure gauges show. Some of them indicate the unit with psi, some others indicate Pa, or kg/cm3, or other uncommon units. Users are often dizz by these pressure units and their conversions. In order to get a correct test result, one has to realize the basics of the principles of pressure and vacuum.
Pressure Unit
"Pascal", referred to as "Pa", which is the most basic metric pressure unit, it defines as one newton per square metre. In case of relatively larger pressure range, in addition to "MPa" (mega Pa), the unit "bar" is also commonly used, which is equivalent to 100,000 pascals. 1 bar = 100000 Pa, "mbar" (millibar) is equivalent to 100 Pa. The other common pressure unit from Imperial units system or United States customary units is psi (pounds per square inch). In Taiwan, most of pressure gauges usually display two units at once, Pa and psi. It is easier to switch between two common systems.

Different Pressure Gauges
Atmospheric Pressure
The unit of atmospheric pressure is atm, which presents the pressure surround the environment. Atmospheric pressure can be measured by pressure gauge, such as a mercury liquid column gauge or a Barometer. The former measure the pressure by the mass of mercury. At both ends of the U-tube of the liquid column gauge, the pressure difference is measured. So, the pressure can be presented in millimetres of mercury height (mmHg). The early Italian scientist measured the atmospheric pressure from such kind of mercury liquid column. A barometer can also be used in atmospheric pressure measurement, which equipped a thin metal plate or metal tube (Bourdon tube) to drive the pointer readings, due to the external pressure. The common gas or liquid pressure gauges belong to this category.
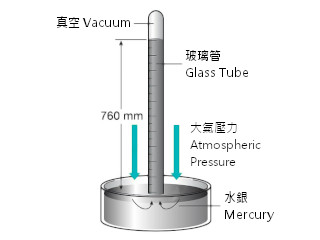
Mercury liquid column with vacuum
However, atmospheric pressure is different at two locations and altitudes. Therefore, atmospheric pressure (1atm) is defined as the average pressure of sea level. The current international definition of 1atm is equivalent to 760mmHg, which is equal to 101325 Pa. Conversions of other common pressure units of atmospheric pressure are 1013.25 mbar, 14.696 psi, 1.033231 kg / cm2, 29.9212 inches Hg of mercury. Torr is also very common. The original definition Torr is 1/760 atm. Since 1atm = 760mmHg was later released by the international standard, it is different from the original 1atm definition. Therefore, in case of scientific labeling, it will be written 1 Torr ≈ 1mmHg , instead of 1 Torr = 1mmHg, however, in practice, the two units can be recognized 'equal'.
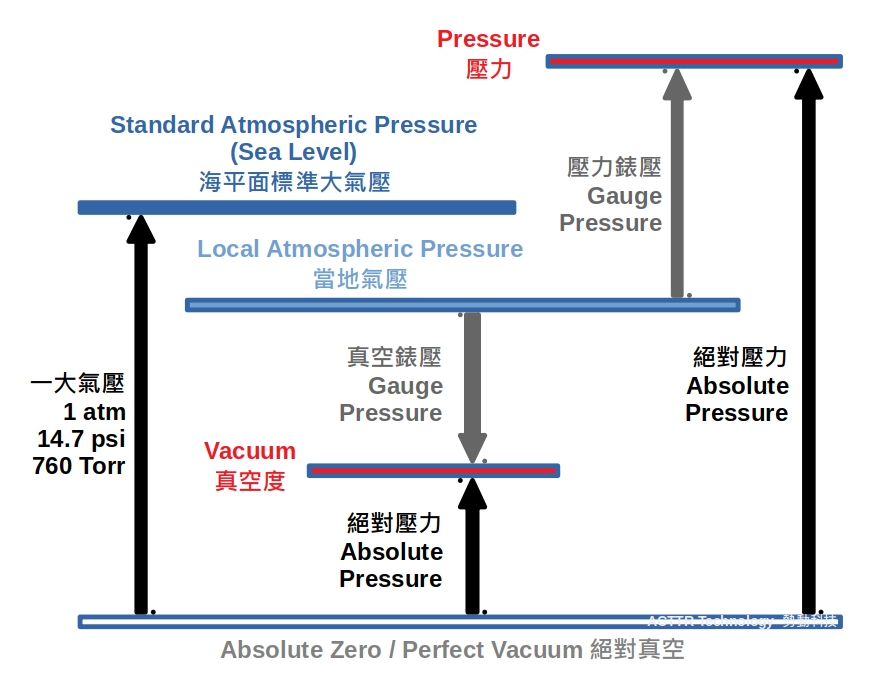
Relative & Absolute Pressure
The measurement of pressure or vacuum is usually in the atmospheric environment. It can be imagined that the measurement result of a pressure gauge or vacuum gauge actually contains the force of atmosphere in the opposite direction, which pushed back the force from the gas to be measured. In other words, the actual pressure of the gas to be measured should have additional 1 atm. The concept is similar to the situation when we carry a cookie bag from ground to the mountain area. The bag will inflate like a balloon, due to the lower atmospheric pressure in the mountain, and applied smaller force against the force of the gas inside the bag.
The result measured by the pressure gauge or vacuum gauge is so called "relative pressure" and "relative vacuum degree", which is also known as "gauge pressure". When the pressure gauge is not ventilated nor fluidized, the meter points at 0. The influence of local atmospheric pressure is ignored and not measured by the gauge. Instead, "absolute pressure" is the result of taking into the local atmospheric pressure in the presentation, which can be expressed by the below formula:
Gauge pressure (Pg) = Absolute Pressure (Pabs) - Local Atmospheric Pressure (Patm)
If the absolute pressure of the measured gas is lower than atmospheric pressure, the pressure should be a negative value. A negative absolute pressure is called vacuum degree, which is equivalent to the following formula:
Vacuum gauge pressure = Local Atmospheric Pressure (Patm) - Absolute pressure (Pabs)
Vacuum gauges are mostly expressed in negative pressure. Before connecting the gauge, the pointer is at the position 0. After connecting it to a vacuum equipment, the negative value is displayed on the gauge. At the end of the scale, it is typically -760mm or -1 kg / cm2 or 100KPa, 0.1MPa (accuratelly, say, 101.325 KPa, 0.101325MPa). Typically, when we describe a vacuum degree, the negative sign can be ignored.
In addition, in the industry or laboratory, the vacuum degree is roughly defined by different levels, as the below table. This table is not an international standard, but a conventional reference:
|
Vacuum Level |
Absolute Pressure (760 Torr + negative |
|
Absolute Vacuum |
0 Torr (basically not existed) |
|
Extreme High Vacuum |
<10E-12 Torr |
|
Ultra High Vacuum, UHV |
1E-9 to 1E-12 Tor |
|
High/Hard Vacuum, HV |
1E-3 to 1E-9 Torr |
|
Medium/Intermediate Vacuum |
25 to 1E-3 Torr |
|
Low/Soft/Rough Vacuum |
760 to 25 Torr |
|
Atmospheric Pressure |
760 Torr |
"Absolute Vacuum" is an ideal theoretical statement. It describes a closed environment where contains absolutely nothing, and the absolute pressure is zero. However, this absolute vacuum environment is basically not existed and not achievable. But "absolute vacuum" is an important concept in representing pressure or vacuum in atmospheric environment. All the names related to the pressure and vacuum in this article can be summarized in the below graph. It makes these definitions easier understanding.