New USP 232 Regulation Coming in 2018!
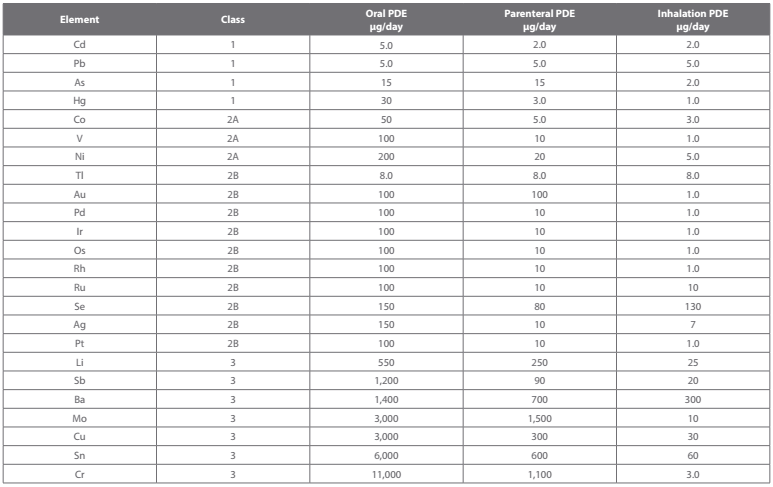
Chapter <232> has now been fully aligned with ICH Q3D Step 4 Guideline, which specifies limits for 24 elemental impurities in drug products, drug substances, active ingredients, and raw materials defined as maximum permitted daily exposure (PDE) levels in µg/day for the three major drug delivery categories. These impurities may be present naturally, derived from the production catalysts, introduced inadvertently through the manufacturing process, or they could be environmental contaminants in the pharmaceutical raw materials. When elemental impurities are known to have the potential to be present, compliance to the specified levels is a requirement.
Additionally due to the ubiquitous nature of arsenic, cadmium, lead, and mercury, those four elements at a minimum, must be monitored. The elemental impurity levels in the drug products, unless otherwise specified in an individual drug product monograph, must show compliance with the limits specified and be made available to the regulatory agency upon request. The full Ulist of elemental impurities is shown in the table below, with Class 1 and 2A elements being the most important to monitor.
ACTTR Technology presents SPEX Certiprep high quality standard products in Taiwan. Providing you with Assurance® level, high reliable standards. All products are traceable certificated. All SPEX CertiPrep standards are certified by ISO Guide 34.
The SPEX CertiPrep USP ICH Pharmaceutical standards provides you the 100% aligned specification. Let you follow the new regulation by the lowest total cost and the highest quality.
The above article was referred to the SPEX Certiperp product DM, and didn't update to the latest announcement of FDA's. All about the information of USP <232> and Q3D guidelines, please refer to the official announcement of FDA, and ACTTR is not responsible to the correction, update, notification duties, regarding to the above article.


