Why Low-Field MR Instruments?
Conventionally, a nuclear magnetic resonance (MR) devices usually equips high-field superconducting magnets. Why are there more and more low-field nuclear magnetic resonance devices available in the market?
Although high-field MR instruments, which are greater than 1.5T, still dominates the market. However, these high-field MR equipments, usually requires a higher end setup and maintenance conditions. In addition, due to its inherent high energy, large volume operation characteristics causes some restrictions in usage.
In contrast, low-field nuclear magnetic resonance instruments have certain advantages in these conditions. Here we come to understand the advantages of the low-field MR instrument, and its advantages.
How A 1.0T Small Animal NMR Brings The Benefits for Preclinical Research?
Magnetic resonance imaging instrument (MRI), is an important equipment in most health centers. It is used to photo the tumor or disease inside the patient's body. MRI has its benefit of non-invasive, zero radiation, image clarity, fast imaging, and other advantages. Compares to X-ray Scanner or Computed Tomography Scanner (CT Scanner, it is also a kind of X-ray machine), MRI Scanner is a very safe instrument. So far, there is nothing hazard to human body was found.
For most of the life science research, biotechnology, pharmaceutical new medicine development, clinical trial applications, MRI is a very ideal device, it can carry out in pre-clinical animal experiments. However, this kind of human body MRI, typically costs million dollars, not to mention the maintenance and consumables, such as liquid helium supplies. For the general laboratory in the university, MRI is an luxury expensive equipment, and it's difficult to equip.
Read more: How A 1.0T Small Animal NMR Brings The Benefits for Preclinical Research?
What Is Crosslink Density? How to Measure It?
Rubber, because of its elastic & waterproof features, is widely used in our daily life and industrial products. Rubber is everywhere, such as soles, laundry gloves, condoms, gasket for pipes, anti-slip feet of furniture, rubber bands, vehicle tires, they are all rubber made. Natural rubber, it is sticky at high temperatures, but it's hard and brittle at low temperatures, in which made this material useless. In order to assign the elasticity, strength and resistance of tearing and corrosion onto rubber, it has to be heated together with sulfur. This chemical process is so called "Vulcanization". In the process, the sulfur atoms links the long molecules of rubber and generates the horizontal bonds between two rubber molecules. So, after rubber is compressed or stretched, after it is relaxed, it restores to its original shape.
What is NMR Spectroscopy?
Nuclear Magnetic Resonance (NMR) spectroscopy is an analytical chemistry technology, for determining the contents or purity of a sample material. It can even be used to analyze the structure of molecular. In the past five decades, NMR spectroscopy has become a superior technology, in the analysis of organic compounds. And NMR had been used by the scientists in the large industry or pharmaceutical companies for many years.
Compare to other spectral analysis methods, NMR spectroscopy is the only one, which is able to analyze and interpreter the entire spectral range and determine the composition of molecular. Although NMR spectroscopy need more amount of samples than mass spectroscopy, however, NMR is a non-destructive testing method. By the modern NMR instrument, it takes less than a milligram of sample material, which is enough for getting good enough data for analysis.
How A NRM Works?
Nuclear Magnetic Resonance (NMR), as the name implies, is a technique for observing the activities of atomic nuclei of moleculars. By analyzing the characteristics of spin of the nuclei, the molecular structure is determined. Since the advent of technology of NMR in the 50's, NMR spectrometers have been used extensively in various research and applications of organic chemicals.
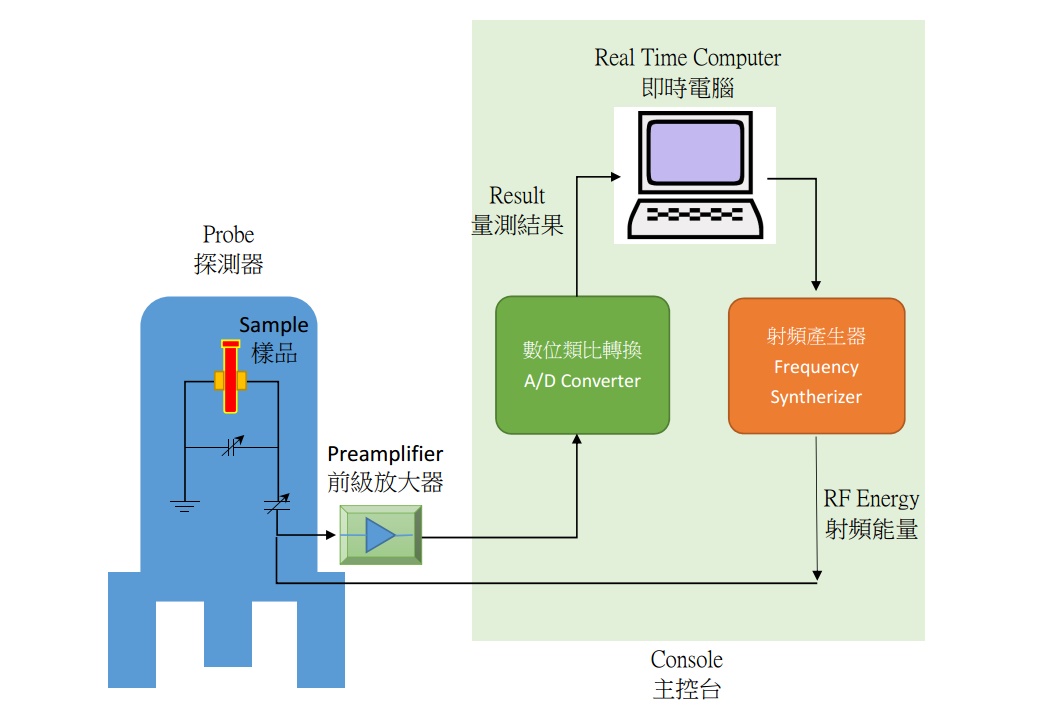
The principle of nuclear magnetic resonance spectroscopy is based on the characteristic of nuclear spin angular momentum. When the nucleus is applied to an external magnetic field, and its spin direction is opposite to the magnetic field, the atomic nucleus will swing around the direction of the magnetic field, as a gyro rotating. This phenomenon, which is so called precession. Precession has its energy, and a certain frequency. Besides, when the nucleus is placed in a constant magnetic field, this frequency will be a fixed value.


